Pulmonary Embolism Transcript
Pulmonary Embolism
This is Dr. Cal Shipley with a review of pulmonary embolism.
Respiratory Physiology and Oxygenation of the Blood
In order to understand the mechanism and significance of a pulmonary embolism, it is critical to be familiar with the physiology of respiration and blood oxygenation in the body. Let’s begin with a review of that.
Oxygen poor-blood flows from the venous system via the vena cava into the right atrium.
Assisted by contractions of the right atrium, blood then passes through the tricuspid valve and into the right ventricle. Oxygen-poor blood is pumped into the main pulmonary artery by the right ventricle. The main pulmonary artery then divides into a left and right branch to distribute the blood to both lungs. The pulmonary arterial system consists of an intricate web of blood vessels, simplified here for graphical presentation purposes. The bronchial tree is an equally intricate system of airways distributed throughout both lungs.
There is a physical interface between the airway system and the pulmonary arterial system, which we’ll take a closer look at in just a moment. To begin the process of blood oxygenation, air is inhaled into the bronchial tree. Let’s zoom in for a closer look to see how the process of oxygen transfer occurs.
The pulmonary arterial system and bronchial tree divide down into ever smaller branches. The smallest arterial branch is known as a pulmonary arteriole.
Tiny airways known as terminal bronchioles give way to even smaller airways known as respiratory bronchioles. At the end of the respiratory bronchioles are structures known as alveolar sacs. Each alveolar sac consists of a number of individual alveoli which we will take a closer look at in just a moment.
The smallest blood vessels, known as capillaries (also pronounced as capillary in Canada and the United Kingdom) originate from the pulmonary arterioles and interface directly with the alveolar sacs.
Taken all together, the capillaries and alveolar sacs are termed the alveolar-capillary complex. Just to give you some sense of the size of these structures, here is the shaft of a typical paperclip which is about one millimeter in diameter. Moving in closer now, we can see the intimate physical relationship between the capillaries and the individual alveoli. I have made the capillaries semi-transparent, exposing the individual red blood cells circulating through them.
The capillary is in close contact with a single alveolus. As noted previously, a respiratory bronchiole is attached to the alveolar sac. Examining a single alveolus and capillary allows a more complete view of their anatomical relationship. The diameter of a capillary is just greater than that of a red blood cell which keeps the red blood cells in close proximity to the wall of the alveolus.
Air enters the alveolus from the respiratory bronchiole. The walls of both the alveolus and the capillary are permeable to oxygen molecules, and allow the movement of oxygen from one to the other. Oxygen molecules attach to red blood cells. Within each red blood cell is an iron-containing protein structure known as hemoglobin, which binds the oxygen molecules. Each red blood cell is typically capable of carrying about one billion molecules of oxygen.
As previously discussed, the freshly oxygenated blood is carried back to the heart through the pulmonary venous system and then ejected into the aorta for distribution to all bodily organs and tissues.
Pulmonary Embolism
Now, we’re ready to take a look at pulmonary embolism. As touched on in our review of physiology, all blood from the venous system returns to the heart via the superior or inferior vena cava.
This, of course, includes the blood returning from the upper and lower limbs. Most pulmonary emboli originate as clots which are formed in the venous system of one of the lower limbs. Although rare, in theory, an embolism may originate from any large vein in the body.
Deep Venous Thrombosis (DVT)
Let’s take a closer look at the venous anatomy of the lower limb. Clots typically form in the deep proximal veins, the popliteal, femoral, or iliac veins. Let’s take a look at the process of clot formation in a vein.
For this presentation, I’m going to use the popliteal vein as an example. And now, a cross-sectional view. DVTs may form anytime there is impaired flow of blood through the vein. This may occur due to venous trauma, venous disease, or when surgery results in localized injury to the veins. Surgeries on the major joints of the lower limb, particularly the hip and the knee, are known to increase the tendency to DVT formation.
In addition, disorders of the coagulation system where there is an abnormally increased tendency to form clots, also known as a hypercoagulable state, may precipitate the formation of DVTs. Hypercoagulability may have many causes and includes both inherited and acquired forms. For an in-depth review of the causes and risk factors for DVT, please click the link located on the lower half of this web page.
Embolization of DVT
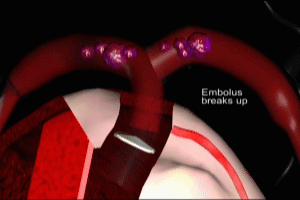
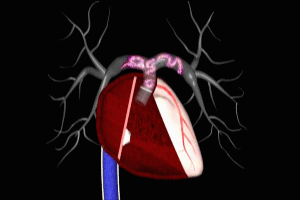
Once the initial DVT has formed it may extend in length by several inches in a matter of seconds. Once the DVT has fully formed, a portion of it may break free and be pushed towards the heart by the venous blood flow. The DVT has now become an embolus, a traveling clot. It follows the course of the vena cava and enters the heart at the right atrium. It is ejected by the right atrium into the right ventricle, where it may often fragment into many smaller emboli.
Each of these fragments is in turn ejected by the right ventricle into the main pulmonary artery. These fragments will flow throughout the pulmonary arterial system until they reach vessels whose caliber is smaller than their own, at which point they will become lodged within the vessel, obstructing local blood flow and interfering with the oxygenation process previously reviewed.
Viewed at the alveolar level, blood within a capillary which is downstream of an obstructing embolus ceases to flow, stopping the movement of oxygen from the alveolus into red blood cells. Just to emphasize the impact on the pulmonary arterial circulation of widespread pulmonary emboli, here’s what normal pulmonary arterial blood flow looks like. Now, contrast that with the dramatic decrease in pulmonary arterial flow which occurs as a result of widespread embolization.
It’s important to note here that the reduction in pulmonary arterial flow is directly related to the number and size of emboli which enter the arterial system. It follows from this then that the physiological impact and survivability of any given episode of pulmonary embolization is also directly related to the number and size of emboli lodged in the pulmonary arterial system.
Surfactant and Alveolar Collapse
In addition, emboli lodged within pulmonary arteries cause localized inflammation, resulting in dysfunction of surfactant. Surfactant is a naturally occurring complex substance which is composed of proteins and fats. It reduces liquid surface tension within lung tissue and prevents alveoli from collapsing during exhalation. Embolism related inflammation spreads to involve alveolar units. With the action of surfactant impaired, areas of lung collapse, also known as atelectasis, further aggravates the issue of reduced blood oxygenation.
Loss of Oxygenation
If the amount of clot lodged in the pulmonary arterial system is sufficient, it may result in a critical impairment of blood oxygenation, leading to multiple organ failure, shock, and death.
Pulmonary Embolism and Pulmonary Vascular Resistance (PVR)
Another physiological consequence of pulmonary emboli is an increase in pulmonary vascular resistance, also known as PVR. This increase in PVR is a direct result of the physical obstruction to blood flow through the pulmonary arterial system caused by the emboli.
The level of PVR at any given moment determines how much force the right ventricle must generate with each contraction in order to push blood through the pulmonary arterial system. In the presence of the increased PVR that occurs with multiple pulmonary arterial emboli, the workload on the right ventricle can be dramatically increased. If the increase in PVR caused by pulmonary emboli results in a workload which is beyond the capacity of the right ventricle, heart failure will occur, with subsequent cardiogenic shock and death.
Death in Pulmonary Embolism
Fatality in pulmonary embolism may occur as a result of acute hypoxemia, or cardiac failure. In many cases, death occurs due to a combination of both factors. Left untreated, the fatality rate of pulmonary embolism is about 30%. This, of course, means that 70% of patients with an initial episode of pulmonary embolism will survive without treatment. This means that in 70% of initial episodes of pulmonary embolism, at onset, the loss of blood oxygenation is not critical and pulmonary vascular resistance has not risen to levels capable of triggering right heart failure.
Therapy of Pulmoary Embolism
As we have seen, the key pathophysiological issue in pulmonary embolism is the abnormal formation and embolization of clots. It makes sense then, that for those who survived the first salvo of emboli, anticoagulation therapy is the most important element of early treatment. While the focus of this presentation is etiology and pathophysiology, anticoagulation therapy plays such a key role in the topic of pulmonary embolism that I’d like to include a few basics on how it works.
Anticoagulation in Pulmonary Embolism
Heparin is the most common drug used to initiate anticoagulation in individuals with new-onset pulmonary embolism. Unfractionated heparin is a naturally occurring polysaccharide, which consists of molecular chains of varying lengths and weights. Unfractionated heparin has a very short half-life. To be effective, it must be administered continuously by an intravenous drip. Daily monitoring of coagulation function via blood tests is essential when using unfractionated heparin.
Fractionated Heparin
Fractionated heparin was first developed in the late 1990s. It was discovered that through an enzymatic process, the molecules of heparin could be cleaved into smaller fragments. These fragments are of very low molecular weight, hence, fractionated heparin is also known as low molecular weight heparin. The chemical characteristics of low molecular weight heparin provide several benefits when compared to unfractionated heparin in the treatment of pulmonary embolism.
Fractionated versus Unfractionated Heparin
Low molecular weight heparin has a longer half-life than unfractionated heparin. This means that it can be dosed just once or twice daily, instead of as a continuous infusion. Unlike unfractionated heparin, low molecular weight heparin may be administered subcutaneously. This allows home administration by patients in scenarios where outpatient treatment is considered appropriate.
Perhaps of greatest importance, low molecular weight heparin has a very predictable correlation between dosage and anticoagulant effect, eliminating the need for regular monitoring of coagulation function. This characteristic further facilitates outpatient treatment for selected patients with pulmonary embolism. Most studies have shown no statistically significant difference between clinical outcomes in patients using low molecular weight versus unfractionated heparin.
In view of these comparable clinical results and its relative ease of administration, low molecular weight heparin has rapidly become the heparin of choice amongst clinicians, for both inpatient and outpatient use.
Heparin Mechanism of Action
Now let’s look at how heparin works as an anticoagulant. In order to better understand how heparin works, let’s do a quick review of the process of blood coagulation in the human body.
Coagulation in the Human Body
Under normal circumstances, initiation of coagulation is triggered by injury to the wall of a blood vessel. Platelet cells are immediately activated to migrate to the site of injury and plug the hole. Simultaneously, the clotting cascade is activated. The clotting cascade consists of the sequential activation of a series of enzymes, also known as factors. The final step in the sequence is the conversion of soluble fibrinogen into insoluble strands of fibrin.
Clot Structure
The fibrin greatly reinforces the strength of the initial platelet plug. This is the essential structure of a blood clot: platelet cells reinforced by interwoven strands of fibrin. The conversion of fibrinogen into fibrin is facilitated by the enzyme thrombin. One step earlier in the sequence, factor Xa interacts with prothrombin to produce thrombin. It is on these components of the clotting cascade that heparin works to inhibit coagulation.
Unfractionated and low molecular weight heparin differ in their actions on the coagulation cascade.
Unfractionated heparin binds not directly to thrombin but rather to antithrombin, a protein which is capable of inactivating both thrombin and factor Xa. This binding increases antithrombin activity against thrombin a thousandfold and provides a similar boost in activity against factor Xa. Low molecular weight heparin also binds to antithrombin, but increases its activity against factor Xa only.
Heparin In Pulmonary Embolism
The key point to take away from this analysis of heparin’s activity in the coagulation cascade, and this applies to both the unfractionated and low molecular weight forms, is that heparin interferes with the formation of clots, and it has no activity whatsoever in dissolving clots which have already formed.
This then begs the question of what use is heparin in the treatment of deep venous thrombosis and pulmonary embolism? The answer is that heparin is used in the hope that it will stop the formation of new clots and prevent the propagation (extension) of existing clots.
This buys precious time, preventing deterioration of the effected individual’s clinical condition due to increased clot burden while the body’s natural clot busting action, also known as fibrinolysis, dissolves existing clots and re-establishes normal blood flow through the pulmonary arterial system, a process known as recanalization. The process of complete recanalization may take from days to months to occur. Some arteries may remain permanently, partially or completely obstructed despite the passage of time.
In spite of adequate heparinization, new clot formation or propagation of preexisting clots may nevertheless occur, in both the limbs or the pulmonary arterial system, often resulting in catastrophic physiological consequences. In addition, even in the absence of new clot formation or propagation of preexisting clot, anticoagulation therapy does not preclude the possibility of further embolization of fragments from a preexisting DVT.
You can learn more about pulmonary embolism by surfing over to my article, Man Runs a Marathon with a Pulmonary Embolism. You’ll find a link to it on this web page.
Cal Shipley, M.D, copyright 2020