Bionic Pancreas and Diabetes Mellitus Review
 Ever since Frederick Banting, and his assistant Charles Best, successfully extracted insulin from a dog’s pancreas in 1921, making possible a practical treatment for diabetes mellitus (also known as “sugar diabetes”), the medical world has been working to more perfectly emulate the function of the human pancreas. Now, in a recent study published in the New England Journal of Medicine, Steven J. Russell and colleagues have taken a look at the effectiveness of the “Bionic Pancreas” in controlling blood glucose levels.
Ever since Frederick Banting, and his assistant Charles Best, successfully extracted insulin from a dog’s pancreas in 1921, making possible a practical treatment for diabetes mellitus (also known as “sugar diabetes”), the medical world has been working to more perfectly emulate the function of the human pancreas. Now, in a recent study published in the New England Journal of Medicine, Steven J. Russell and colleagues have taken a look at the effectiveness of the “Bionic Pancreas” in controlling blood glucose levels.
Diabetes Mellitus – What Is It?
First, a brief review of what diabetes is. Simply put, diabetes mellitus, is a condition in which the body has lost control of blood glucose levels.
Glucose
Glucose is a chemical essential to human life. It is a key energy source for the bodily tissues and organs (particularly the brain). It is also used as a chemical precursor to several important substances including starch, cellulose, vitamin C (ascorbic acid), and glycogen (not to be confused with glucagon – more later).
Glucose may also be added to proteins and lipids (fats) in a process known as glycosylation.
Hypoglycemia and Hyperglycemia
Maintenance of glucose levels within a certain range is critical to normal bodily function. Too little glucose in the bloodstream (hypoglycemia), and organs like the brain will shut down their functions very quickly.
In the short term, abnormally high levels of blood glucose can cause severe upsets in metabolic processes, and dangerous acid-base and electrolyte imbalances.
In the long-term, chronically elevated blood glucose levels markedly accelerate the rate of hardening of the arteries (atherosclerosis), mostly as a result of deposition of excess glucose molecules in the walls of arteries.
Diabetes Type-1 and Type-2
There are 2 broad categories of diabetes: insulin dependent Type 1 diabetes, and non-insulin dependent Type 2. For the purposes of our discussion today, we will be focusing on Type 1, insulin-dependent diabetes.
How does the body control blood glucose?
From the preceding, it can be fairly guessed that keeping the level of blood glucose within the normal range is very important. Enter: the Pancreas. The pancreas is in control of blood glucose levels in the human body. It does so by secreting either insulin (to lower glucose levels), or glucagon (to raise glucose levels), from specialized groups of cells known as the Islets of Langerhans (named for the scientist who first discovered the Islets in 1869). The islets, (and therefore the glucose controlling tissue of the body) make up only about 1-2% of the total mass of a typical human pancreas, so a critically important aspect of human physiology is packed into a very small space.
The pancreas is stimulated to secrete insulin and glucagon by elements of the central nervous system, which provide a continuous monitoring of blood glucose levels. This provides for a system that can respond rapidly and yet smoothly, to even small changes in glucose levels, literally in seconds, with either insulin or glucagon being secreted in constantly varying amounts.
Exogenous (external source) insulin for Diabetes treatment
While the Islets of Langerhans were discovered in 1869, their significance at that time were unknown. Subsequent researchers during the latter half of the 19th century eventually put together the idea that the pancreas was in charge of blood glucose control.
Best and Banting
But it took until the 1920s, when Best and Banting first extracted insulin from a pancreas, for commercially usable forms of insulin to become available. And initial treatment with exogenous insulin, while it helped to control glucose levels in diabetic patients, was fraught with potentially severe allergy side-effects, owing to the chemical impurity of early extracts.
Exogenous Insulin
For all its early problems, the availability of exogenous insulin revolutionized the treatment of diabetes. Rather than having abbreviated lifespans filled with premature onset of atherosclerosis, organ failure, and an early death, insulin-dependent diabetic patients could get control of their disease and (in some cases at least) live out a normal or near-normal lifespan.
Limitations of Exogenous Insulin
While the availability of insulin for injection caused a paradigm shift in the treatment of insulin-dependent diabetes, as time went on, physicians discovered that there were still problems cropping up in diabetic patients on insulin.
The problem was really very simple – giving yourself an insulin injection 2, 3 or even 4 times a day, provides crude control of blood glucose levels compared to the second-to-second command and physiologic control provided by the human pancreas and nervous system.
And while the use of extracted insulin was a lifesaver in the short-term for diabetic patients, virtually eliminating the potentially severe short-term complications of unregulated blood glucose, in the longer- term, many patients continued to have premature onset of diseases related to arterial hardening (atherosclerosis), such as blindness, kidney (renal) failure, heart attacks (myocardial infarction), and decreased lifespan. Study after study indicated that diabetic patients with “tight” control of blood glucose levels, that is, less variation of their glucose levels outside the norm, and particularly those with less elevation of their glucose above the normal range, lived longer and had fewer complications than those whose control was not as “tight”.
Blood Glucose Control
It all boils down to trying to achieve that tight blood glucose control. For some diabetes patients, tight control is not an issue. For others, it’s a nightmare. These folks are so-called “brittle” diabetics. And it’s not for lack of trying. Even the most conscientious Type 1 diabetics on daily multi-injection regimens can have periods of wildly fluctuating glucose levels.
Insulin development for diabetes
The development of insulins with varying durations of activity (short, medium and long-acting) in the decades following Banting and Best, was a major advance in diabetes therapy. Patients could now use a combination of insulins of differing duration and onset of glucose lowering activity, providing for smoother control and fewer fluctuations in glucose levels.
The synthesis of recombinant human DNA based insulin in the 1960s virtually eliminated the allergy and insulin resistance issues associated with animal derived products. Both of these developments improved the ability to control blood glucose levels in many patients.
But in spite of these advances, problems continued for brittle diabetics. So scientists began to bend their minds toward perfecting an imitation of pancreatic function.
The Insulin Pump
The Insulin Pump was the next step in emulating the function of the pancreas.
Basically, an insulin pump is a reservoir of short-acting insulin, contained within a pager-sized unit (that can be clipped onto a belt. A small catheter extends from the unit, and ends in a small hollow needle, which is inserted beneath the skin, and provides for flow of the insulin subcutaneously. Typically, the insulin pump continuously emits a small amount of insulin, 24 hours a day, and then the patient pushes a button telling the pump to issue a burst of insulin, usually right before eating (and taking on a glucose load).
Commercial use of insulin pumps for the treatment of insulin-dependent diabetes started in the 1970s. Early models were bulky and basic in operation, but modern units have programmable settings that permit variation of continuous insulin emission rates as well as customization of bolus dosing, and most recently, the ability to interact and respond to a glucometer in real time.
Hypoglycemia in Type 1 Diabetes treatment
The progress in diabetes treatment with newer insulins and insulin-pumps has made tighter control for many Type-1 diabetics a reality, with all of the benefits that acrue when blood glucose levels can be controlled in a more physiologic way (ie – more like the pancreas!).
But with tighter control comes another problem – the potential for hypoglycemia (abnormally low blood glucose levels). In diabetic patients, it is considered ideal to continuously maintain blood glucose levels in the low 100s, preferably around 100-110 mg/dl. Symptoms of hypoglycemia tend to start occurring around the 50-60 mg/dl level, with severe neurological disturbances, or loss of consciousness, occurring with levels below 50 mg/dl.
Many variables contribute to the level of blood glucose in a human being at any given moment. Variations in diet, sleep and activity levels, as well as minor viral illnesses can cause unexpected fluctuations in glucose levels. The same daily amount of insulin that has worked well for weeks or months, may suddenly cause hypoglycemia, with all of its attendant discomforts and dangers.
Two Problems with Synthetic Insulin
So even with synthetic insulins and insulin pumps, there were still two problems in the struggle to imitate the pancreas.
The first problem was that there was not a tight integration between blood glucose levels and introduction of insulin into the body; nothing like the physiologic second to second monitoring and response of pancreas and nervous system.
The second problem was that, while synthetic insulin can lower blood glucose levels, it cannot raise them if they get too low. In other words, there is no way for insulin injections or an insulin pump to resolve hypoglycemia. In the human body, the pancreas secretes the hormone glucagon to raise blood glucose levels.
Diabetes and the Bionic Pancreas
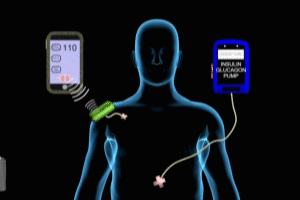
So now onto the ultimate artificial pancreas, the “Bionic Pancreas” (while technically an apt name for the electronic artificial pancreas, the term “Bionic Pancreas” seems to conjure images of a pancreas that can walk, talk, and perhaps beat you in the 100 yard dash!).
Bionic Pancreas Components
The bionic pancreas consists of several parts: 1) a module that can pump insulin 2) a module that can pump glucagon 3) a software program that contains the mathematical instructions (algorithm) to monitor glucose levels, and respond to them and 4) a continuous operation glucose monitor that can record blood glucose levels. (Dr. Russell’s group used a Dexcom G4 Platinum – an ingenious device that is able to accurately measure blood glucose levels via a subcutaneously placed probe – more information on the G4 here)
Bionic Pancreas System Setup
In the recent Steven Russell study published in the NEJM, the system was setup to respond to blood glucose levels every 5 minutes 24/7, with the software program deciding whether to give insulin or glucagon at predetermined levels. For example, if glucose levels dropped to less than 65 mg/dl in the bloodstream, then a dose of glucagon was given, if higher than 110, insulin was given instead. For the Russell study, the software program resided in an iPhone, which communicated with the glucometer and the insulin/glucagon pumps wirelessly.
To give the system a heads-up that a glucose load was coming (what we call eating), patients pushed a virtual button on their cellphone announcing their intention to eat a meal, and they could further specify the size of the meal, so that the Bionic system could emit an appropriate pre-meal “priming bolus” of insulin to prevent post-meal glucose levels from jumping too high.

Did the Bionic Pancreas work?
Was the Bionic Pancreas better at emulating human physiological control of blood glucose levels?
The Russell study looked at blood glucose levels in insulin-dependent diabetics who were already using insulin pumps to treat their diabetes. Study subjects used insulin pumps for 5 days, then the Bionic Pancreas for 5 days, and blood glucose levels results were compared. Overall, the Bionic Pancreas delivered statistically significant improvements in glucose level control compared to an insulin pump.
Russell is careful (and wise) to state some of the possible limitations of his study, and the effect these may have on his conclusions. But if the Russell data are valid, another exciting chapter in the treatment of insulin-dependent diabetes may be just over the horizon.
To review the Russell Bionic Pancreas Study on NEJM, click here. To read more about Steven J. Russell, M.D., and his fascinating bionic pancreas development project, click here.
Cal Shipley, M.D. copyright 2020
