Cardiac Tamponade
 Cardiac Tamponade: Definition
Cardiac Tamponade: Definition
Simply stated, cardiac tamponade occurs when the chambers of the heart (ventricles and atria) are compressed by an accumulation of fluid between the heart and the pericardial sac (also known as the pericardium).
In an article in the New England Journal of Medicine this past December, Martin M. LeWinter, M.D. discusses one of the possible causes of cardiac tamponade: acute pericarditis (“Acute Pericarditis” NEJM December 18th, 2014). Acute pericarditis refers to a realtively rapid onset of inflammation (irritation) of the pericardium. Dr. LeWinter begins by presenting the case of a previously well 25 year-old male patient who presents with a 3 hour history of left sided chest pain, complaints typical of acute pericarditis. The case presentation provides a nice segue into the very succinct review of acute pericarditis that follows.
After the case presentation and introduction to the article, Dr. LeWinter summarizes a few key points regarding acute pericarditis: 1) the inflammation in 80-90% of cases is of unknown cause (idiopathic), and presumed to be viral 2) 70-90% of cases resolve completely without further complications.
On the other side of the causation coin, in some studies, up to 14% of patients experiencing acute pericarditis will go on to develop a significant abnormal accumulation of fluid (also known as a pericardial effusion) between the pericardium and the heart, resulting in cardiac tamponade.
Cardiac Tamponade: Anatomy of the Pericardial Sac (Pericardium)
The pericardium consists of a 2 layered sac which envelops the heart, and the great vessels (at their origins from the heart). The 2 layers are known as the visceral layer, and the parietal layer. The visceral layer is quite thin and is closely applied to the epicardium (outer layer of the heart) and the great vessels (aorta, superior and inferior vena cava, and pulmonary arteries and veins). The outer parietal layer is much thicker (approximately 1mm) and is composed primarily of collagen. The parietal layer is attached to the manubrium and xyphoid portions of the sternum (breastbone), the diaphragm, and the vertebral column, and these attachments provide stability for the position of the heart within the thorax (chest). Other functions for the pericardial sac have been proposed, including as a barrier to infection from adjacent thoracic structures, lubrication of the surface of the heart, augmentation of atrial (small chambers) filling, and maintenance of normal pressure-volume relationships of the ventricles (large chambers) and atria. Removal of the pericardium appears to have little long-term adverse effect.
There is a narrow potential space between the 2 pericardial layers. Under normal circumstances, this potential space holds between 15-60 ccs of fluid. Quantities of fluid greater than these normals are referred to as a pericardial effusion. As the quantity of fluid in the pericardium increases, the heart undergoes compression. If effusion size continues to increase, a critical threshold is eventually reached at which cardiac output is severely compromised. This process is said to occur in 3 stages: 1) fluid filling the pericardial potential space, followed by 2) fluid accumulating faster than the parietal pericardium’s ability to stretch and 3) pressure on the right ventricle which reduces right ventricular filling volume. This results in lower filling volume of the left ventricle, and hence, lower cardiac output. The rate at which excess fluid accumulates is very important to the presence or absence of tamponade. Rapidly increasing effusions may cause tamponade with as little as 100-200ccs of accumulation, while more slowly progressive effusions give the pericardial sac more chance to stretch. In these situations, tamponade may not occur until as much as 1500cc of effusion has collected.
Cardiac response to these changes initially is to increase heart rate in order to compensate for lower output. If nothing is done to treat the effusion, decompensation and cardiac arrest will occur.
Cardiac Tamponade: Causes
Pericardial effusion which underlies tamponade may consist of typical pericardial fluid, blood, pus gas or a combination of these. As mentioned above, the rate at which effusion accumulates determines the rapidity with which tamponade may occur, therefore, it is useful to divide tamponade causes into acute (rapid onset) and sub-acute or chronic (slower onset). The term “pericarditis” refers to a state of inflammation occurring in the pericardium. This condition is often associated with abnormal production of pericardial fluid (effusion), particularly in the sub-acute and chronic causes of tamponade.
Acute causes of cardiac tamponade
Penetrating Trauma – such as stab wounds to the chest, may pierce the pericardial sac or cardiac chambers or great vessels resulting in hemorrhage into the pericardial sac (hemopericardium) and subsequent tamponade. Blunt force trauma, most commonly as a result of motor vehicle accidents, may also result in hemo-pericardium and tamponade.
Aortic dissection – approximately 7000 cases of aortic dissection are recognized annually in the United States. The results of this disease may be catastrophic and fatal if not recognized and treated very early in the course. The aorta is made of 3 tissue layers – an inner intimal layer, a middle or medial layer, and an outer, or adventitial layer. Dissection is thought to occur due to a small tear in the intimal layer, which exposes an already diseased medial layer to the propulsive force of blood being ejected into the aorta from the left ventricle. Blood enters the intimal tear, and cleaves the medial layer longitudinally. In some cases, the blood will eventually be forced through the adventitial layer, resulting in acute hemorrhage. Aortic dissections are classified according to the portion of the aorta in which they occur: the ascending aorta (as it comes from the heart) , the aortic arch, the descending aorta (the portion immediately following the arch), or the abdominal aorta. 65% of aortic dissections occur in the ascending aorta. The first several centimeters of the ascending aorta are enclosed by the pericardial sac, therefore, if an adventitial rupture should occur, hemorrhage into the pericardial sac may cause rapid onset of tamponade.
Following treatment of MI (myocardial infarction or heart attack) with thrombolytics (clot-busting agents) and/or heparin due to myocardial rupture and/or hemorrhagic pericarditis (bleeding from the pericardial sac itself)
Iatrogenic (physician caused) – during central line or pacemaker insertions, great vessels or cardiac chambers may be injured with subsequent bleeding and tamponade. After coronary bypass surgery, graft rupture can lead to tamponade. During angioplasty, coronary artery rupture may precipitate tamponade.
Sub-Acute or Chronic causes of cardiac tamponade
Malignancy – has become a leading cause of pericardial effusion in developed countries. 10% of all cancer patient will develop cardiac tamponade. The most common mechanism is direct implantation of tumor into the pericardium, although effusion may also occur from obstruction by tumor of mediastinal lymph nodes. Lung cancer is the most commonly seen malignancy, accounting for %40 of malignant effusions. Lymphomas and breast cancer may also be the cause. HIV-related effusions are increasing with the incidence of the disease, and are associated with Kaposi’s sarcoma and lymphomatous pericardial involvement.
Viral pericarditis (pericardial inflammation) – viruses are the most common cause of infectious pericardial effusion and subsequent tamponade. Effusion may result from direct viral damage or secondary immune system response. Coxsackie and Echo viruses are the most commonly isolated agents. Cytomegalovirus has a predilection for individuals whose immune systems are deficient (immunocompromise).
Bacterial pericarditis – is generally characterized by a purulent (pus) effusion. The most common agents are streptococci, staphylococci and pneumococci. Infection typically spreads to the pericardial sac from pneumonia in the lungs or empyema (infection of the pleural coverings of the lung), but may also arise from blood borne infection (septicemia).
Fungal pericarditis – is rare. Most commonly caused by Histoplasma, but has also been reported to occur with Cryptococcus, Blastomyces and Pneumocystis carinii (PC – occurs frequently in HIV-infected individuals). Drug addicts, individuals with immunocompromise, and those taking corticosteroids or long-term broad spectrum antibiotics are particularly susceptible to opportunistic fungal infections.
Tuberculous pericarditis – is most common in the third world, particularly Africa (with HIV infection), and in immunocompromised individuals. Spread of TB to the pericardium typically occurs from involved peritracheal, peribronchial or mediastinal lymph nodes, but may occasionally spread directly from adjacent lung lesions.
Uremia – the incidence of hyper-uremic pericarditis has been markedly reduced since the advent of renal dialysis. The specific mechanism by which pericarditis occurs as a result of high levels of blood urea nitrogen (BUN) and Creatinine are unclear. Large, slowly accumulating effusions are typical.
More commonly seen today is a form of dialysis-related pericarditis in patients whose BUN and Creatinine levels are normal or only slightly elevated. Again, the cause is uncertain.
Radiation – therapeutic radiation to the mediastinum may trigger pericarditis and effusions. Breast cancer, Hodgkin Disease and non-Hodgkin lymphomas are the most commonly involved malignancies. Pericardial injury from radiation is related to the dose, duration and type of radiation administered, as well as the area of pericardium exposed. Approximately 2% of therapeutic radiation patients develop pericarditis. Radiation pericarditis may occur during therapy as an acute illness with chest pain and fever, or manifest as a delayed form 1-20 years after treatment. Cardiac tamponade is possible with either form.
Collagen vascular diseases and autoimmune causes – pericardial effusion and eventual cardiac tamponade may be seen in any autoimmune disease, but the most commonly related are Rheumatoid Arthritis, Systemic Lupus Erythematosus (SLE), and progressive systemic sclerosis (scleroderma). The auto-immune diseases are all characterized by bodily production of antibodies targeted against healthy native tissues. For reasons that are as yet not determined, the immune system erroneously “sees” certain tissues of the individual in the same way as it “sees” a foreign invader, such as a virus or bacteria. The autoimmune diseases are classified by the tissues targeted, for example, in Rheumatoid Arthritis, joint space cartilage is targeted for destruction. Pericardial effusions in individuals with auto-immune diseases is typically inflammatory in nature, usually containing high concentrations of white blood cells. Disease-specific antibodies are often found.
Pharmaceuticals – A number of prescription drugs have been implicated in cases of pericardial effusion and tamponade. In most cases, this phenomenon is related to a drug-triggered SLE syndrome.
Idiopathic (unknown cause)
Cardiac Tamponade: Symptoms and Signs
Physical complaints with cardiac tamponade may be very non-specific. These may include chest pain, cough, dyspnea (shortness of breath) and tachypnea (rapid breathing) Beck described the classic triad of physical signs: hypotension (low blood pressure), distention of the jugular veins of the neck (with elevated central venous pressure), and quiet or muffled heart sounds. These signs may not be present if the tamponade has come on rapidly. The presence of pulsus paradoxus (a fall in systemic arterial pressure of 10mm Hg or more with inspiration) should raise suspicions of tamponade. A pericardial friction rub, caused by a swollen pericardium coming into contact with the chest wall, may be heard.
A detailed history is extremely important in the diagnosis, as pre-existing conditions such as malignancy, auto immune disease or therapeutic radiation to the chest area, etc. predispose individuals to pericarditis and hence tamponade.
Differential Diagnosis
Other conditions in which Pulsus Paradoxus, hypotension and jugular venous distention may be present include Pulmonary Embolism, COPD (Chronic Obstructive Pulmonary Disease or Emphysema), constrictive pericarditis, restrictive cardiomyopathy and right ventricular infarction.
Cardiac Tamponade: Diagnosis
Chest xray – will show an effusion only if the fluid accumulation is >200cc.
Echocardiogram – can make the diagnosis when an effusion is seen in combination with right atrial and ventricular collapse during diastole, (relaxation phase of the ventricles).
Computed Tomography (CT) – CT can be very effective in defining the presence and extent of pericardial effusion, and is generally more accurate than ultrasound in differentiating pericardial and pleural effusions. However, CT scanners lack the portability of an ultrasound machine, and requires the patient be transported to the CT machine, and so patient stability must be taken into account.
EKG – classic finding is electrical alternans in which the axis of the QRS complex alternates with each beat (SEE ILLUSTRATION). In addition, generalized low voltages may be seen throughout the tracing, and diffuse ST segment elevations may be seen if acute pericarditis is present.
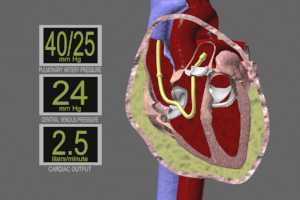
Thermodilution catheter (Swan-Ganz pulmonary catheter) – readings can confirm the diagnosis. Typical findings show equalization of pressures during diastole in the pulmonary artery, right ventricle, right atrium and intrapericardium. These pressures normally show a difference of 15-30mm Hg.
Laboratory tests – are not helpful in initial diagnosis, but electrolytes, BUN, creatinine, ESR, thyroid function tests, ANA, RF, PPD, blood cultures, viral titers, and pericardial fluid analysis and cultures should all be performed to help determine underlying causes.
Cardiac Tamponade: Treatment
Cardiac tamponade is a medical emergency and is associated with a high rate of mortality. Urgent and aggressive measures are therefore essential. Large bore intra-venous lines should be initiated to permit fluid infusion (blood, saline, or dextran) at a high rate. This increases overall circulatory volume, and hence, increases filling pressure into the right ventricle to assist the heart in overcoming pericardial effusion compression. Drugs that improve cardiac output (inotropic) and stabilize blood pressure (vasopressors) may be utilized.
Pericardiocentesis
Pericardiocentesis is the definitive therapy for acute cardiac tamponade. If possible, this procedure should be performed under ultrasound guidance. Ultrasound can define the location of greatest fluid accumulation, as well as its relationship to the body wall. This information is invaluable in choosing the best entry site and angle of penetration fro fluid removal.
Pericardiocentesis involves the insertion of a needle into the pericardial space, and then withdrawal of the fluid via an attached syringe. A catheter may be left in place after initial fluid removal to permit drainage of any further fluid accumulation.
There are three approaches used:
Parasternal – where the pericardial needle is inserted along the left side of the sternum (breastbone) in the (intercostal) space between the fifth and sixth ribs. It is important that the needle be inserted immediately lateral to the sternal border, so as to avoid injury to the internal mammary artery. There appears to be a higher incidence of pneumothorax (collapsed lung) with this approach compared to the subxyphoid approach.
Subxyphoid – the needle is inserted between the xyphoid process (the lowest portion of the sternum) and the left costal (rib) margin, at an angle of 30-45 degrees to the skin, with the tip of the needle aimed at the left shoulder or sternal notch.
The subxyphoid approach has been the most commonly used traditional approach, but has lost favor in recent years due to a higher rate of injury to the cardiac chambers, especially the right atrium, compared to the subxyphoid approach.
Apical – in the less commonly used apical approach, the needle is inserted 1cm lateral to, and on the intercostal space just below the apical beat. The needle tip is aimed toward the right shoulder. The theoretical advantage of this approach is based on the fact that the coronary vessels are small at the apex, and the wall of the left ventricle in this area is thick, so that if the needle inadvertently penetrates the ventricular wall, it is more likely to seal off the injury.
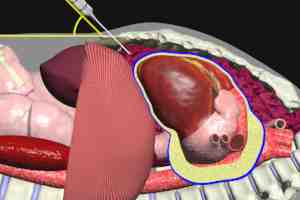
Punctures of the lungs and cardiac chambers (more frequently the right ventricle), and lacerations of the great vessels may occur as a result of pericardiocentesis. These complications are much less frequent in ultrasound guided procedures. Cardiac arrest and death are extremely rare with guided procedures. When pericardiocentesis is performed blindly (without imaging guidance) a higher rate of cardiac arrest and death have been associated, but with the wide availability of ultrasound guidance in American Hospitals, blind procedures are generally restricted to those individuals who are already in cardiac arrest.
EKG monitored pericardiocentesis – in instances where ultrasound or CT imaging is unavailable, or where the patient is already in extremis (ie- in cardiac arrest) necessitating an immediate procedure, EKG monitoring may be used to help avoid injury to the cardiac chambers. After the needle has punctured the skin, but before it penetrates the pericardial sac, one of the “V” leads of the EKG machine is attached to the base of the needle. The “V” lead is then monitored continuously as the needle is advanced. If the needle comes into contact with the epicardium (outer wall of the ventricle) a current-of-injury pattern is seen, consisting of a wide QRS complex with ST elevation. It is critical that an EKG machine and not a cardiac monitor be used, as monitors typically have lower frequency response, and may not show the abnormal complex.
A Note on hemorrhagic cardiac tamponade
Pericardiocentesis is never considered the definitive treatment for hemorrhagic cardiac tamponade (such as caused by stab wounds). Although removal of bloody fluid via pericardiocentesis may bring about temporary relief of signs and symptoms, blood almost always reaccumulates, For this reason, these individuals all require an operative solution involving surgical repair of the injury.
Cardiac Tamponade: Other Treatments
In situations where pericardial effusions reaccumulate, in hemorrhagic pericardial effusions, or where pericardiocentesis has failed to relieve tamponade for any reason, exploratory thoracotomy (opening of the chest) may be employed to 1) open the pericardial sac to permit drainage 2) repair any sources of bleeding, and/or 3) perform partial (pericardial window) or total removal (pericardectomy) of the pericardial sac to prevent reaccumulation of fluid in chronic effusions.
Cal Shipley, M.D.
