Myocardial Infarction Review
DEFINITION OF MYOCARDIAL INFARCTION
Myocardial Infarction involving the left ventricle
Myocardial Infarction (acute MI) refers to the injury or death of heart muscle (cardiac myonecrosis) due to inadequate blood flow (perfusion) from the coronary arteries. Impaired perfusion to the myocardium results in a critical reduction of nutrient supply (primarily oxygen) to the myocardium, a condition known as iscehmia.
EPIDEMIOLOGY OF MYOCARDIAL INFARCTION
Estimates are that approximately 1.2 million people in the United Stated experience a fatal or non-fatal acute MI each year. Approximately every minute, an American dies of an acute myocardial infarction. Coronary artery disease is the leading cause of acute MI, and causes or contributes to about 650,00 deaths annually. Half of all coronary artery disease related-deaths arise from acute MI, and occur within 1 hour of onset of symptoms.
CAUSE (ETIOLOGY) OF MYOCARDIAL INFARCTION
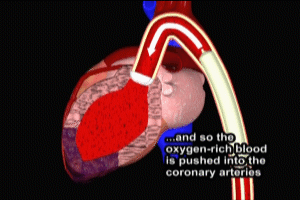
The most common cause of myocardial infarction is related to the formation of a blood clot (thrombosis) in a coronary artery. These coronary clots (thrombi) form at areas of atherosclerotic plaque buildup in artery walls (“hardening of the arteries”).
Typically, rupture or fissuring of plaques initiates the process of thrombosis. Exposure of the internal constituents of plaque (lipid, tissue factor and collagen) to elements of the blood stream cause platelets to adhere and aggregate to plaque. Clot-forming (thrombogenic) blood factors, such as thrombin, are released, as well as substances which cause spasm and narrowing (vasoconstriction) of the affected coronary artery. Ultimately, a clot (thrombus) forms, which either partially or completely obstructs coronary artery flow beyond the point of the thrombus (acute occlusion). Depending on the degree and location of coronary occlusion, inadequate perfusion to a portion of the heart muscle (myocardium) may occur, which may subsequently result in myonecrosis, left ventricular function impairment, severe cardiac rhythm disturbances, and death.
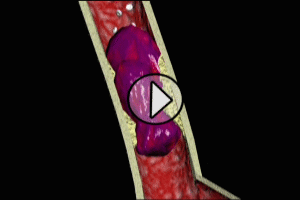
CAUSES OF CORONARY OCCLUSION OTHER THAN ATHEROSCLEROSIS (HARDENING OF THE ARTERIES)
While most MIs are related to atherosclerosis, coronary occlusions may result from clots (emboli) which travel to the coronary artery from other parts of the body (embolism), primary arterial spasm, arterial inflammation (vasculitis), degenerative arterial disease, diseases of the aorta, congenital arterial abnormalities and trauma.
Causes not directly related to coronary artery occlusion
Disease states in which the myocardial oxygen demand is increased may precipitate acute MI in the absence of acute coronary occlusion. Examples of such states include Aortic Stenosis (narrowing of the aorta), insufficiency of the aortic valve, hypertension(high blood pressure) with severe left ventricular hypertrophy (enlargement of the left ventricle), abnormally high levels of thyroid hormone in the bloodstream (thyrotoxicosis), carbon monoxide poisoning, shock, hyperviscosity syndromes (for example, severely elevated red blood cell count as may occur with “blood doping”, pheochromocytoma (abnormally high production of adrenaline), and methhemoglobinemia (presence of large amounts of methemoglobin in red blood cells. Methemoglobin does not bind oxygen, and may therefore lead to myocardial ischemia).
Myocardial Infarction not related to coronary artery disease may also occur in the setting of viral myocarditis (inflammation of the heart muscle), stress-related cardiomyopathy (Takostubo syndrome), and hyper-coagulable (clotting) disorders.
DIAGNOSIS
The Third Universal Definition of Myocardial Infarction, developed by a multinational global task force, and released in 2012, gives the following 5 criteria for the diagnosis of myocardial infarction, is the detection of a rise and/or fall of cardiac biomarker values, with at least one of the values being elevated. The troponin biomarker cTN is the preferred biomarker. In addition, at least one of the following 5 diagnostic criteria should be present:
- Symptoms of ischemia
- New (or presumably new) significant ST/T wave changes or left bundle-branch block (LBBB)
- Development of pathological Q waves on ECG
- Imaging evidence of new loss of viable myocardium or regional wall motion abnormality
- Identification of intracoronary thrombus by angiography or autopsy
SERUM MARKERS
In recent years, the increasing sensitivity and accuracy of measurement of serum markers in acute MI have made them the gold standard for diagnosis of this problem. Serum markers for acute MI, ideally, are substances which are not normally present in the bloodstream, are released by dying myocardial cells, and which are not found elsewhere in the body. The troponin markers cTnT and cTnI are considered the best of these, although the previously discovered creatine kinase MB isoenzyme (CK-MB) continues to be useful.
The troponins (cTnT and cTnI) are contained in the cells of the myocardium, and are chemically distinct (DNA) from their counterparts in non-cardiac muscle. The troponins generally are first detectable 2 to 4 hours after the onset of acute MI, are maximally sensitive at 8 to 12 hours, peak at 10 to 24 hours, and persist for 5 to 14 days. Troponins are both more specific for myocardial injury than CK-MB, and also more sensitive. Even small acute Mis can cause a many fold increase in troponin levels.
One disadvantage to the troponins is that, due to their prolonged elevation and persistent serum levels, recurrent MIs, or extension of the affected myocardial area after the initial injury may be difficult to detect.
The MB isoenzyme of creatine kinase (CK-MB) is more specific to myocardial injury than total serum levels of CK, however, the specificity is improved by calculation of an MB/total CK ratio. However, even with ratio calculation, CK-MB is not as specific as either Troponin marker for myocardial injury. As a result, CK-MB is primarily used to confirm acute MI with an already positive Troponin level in a unclear clinical situation, to look for evidence of re-infarction during the days after initial MI, or when Troponin measurement is not available.
It is important to note that the finding of an elevated level of either Troponins or CK-MB does not identify the underlying cause of myocardial infarction.
CK-MB increases within 3 to 4 hours after the onset of acute MI, is maximally sensitive within 8 to 12 hours, peaks at 12 to 24 hours, and returns to normal in 2 to 4 days.
Other lab testing
A complete blood count, platelet count, coagulation studies (partial thromboplastin time – APTT, prothrombin time – PT), serum glucose, kidney function and electrolytes, and lipid panel should all be performed upon admission to hospital. Typically, the white blood cell count rises within a few hours of acute MI and persists for 3-4 days. Coagulation studies are useful particularly if fibrinolytic (clot “busting”) therapy is contemplated.
MYOCARDIAL INFARCTION: PRESENTING SYMPTOMS
The most common presenting symptom of myocardial infarction is chest pain. Classically, the location of the pain is described as being behind the breastbone, and may be described as a feeling of heaviness, squeezing, band-like, crushing or burning in quality, However, the location of the pain may vary considerably, being present in the left chest, the right chest, or all across the chest. Highly localized sharp or stabbing pain is not characteristic.
Radiation of the chest pain is typically to the left arm, but may also radiate to the right arm, both arms, into the neck jaw or teeth, upper abdomen (epigastrium) or between the shoulder blades. Pain noted below the umbilicus of the abdomen or above the jaw is not characteristic of acute MI. Chest pain which is aggravated or reproduced by external pressure on the chest by the examiner is not characteristic.
Additional symptoms may include one or more of the following: nausea, vomiting, profuse sweating (diaphoresis), cardiac palpitations (perception of abnormal heartbeat), vomiting, lightheadedness, restlessness, weakness, or anxiety.
Chest pain related to coronary ischemia without MI is known as angina. The chest pain of angina is typically of lesser severity and shorter duration than that of acute MI. Ischemic related chest pain that lasts 20-30 minutes or more must be considered to be caused by acute MI until proven otherwise.
Approximately 20% of acute myocardial infarctions are completely asymptomatic (symptom-free), and may first be discovered as old events on routine EKGs performed for other purposes.
MYOCARDIAL INFARCTION: PHYSICAL EXAMINATION
Physical examination may be completely normal during acute MI. There are no specific physical signs which will make the diagnosis. Blood pressure may be elevated, normal or low. Pulse may be reduced, normal or elevated. Mis involving the anterior (front) wall of the left ventricle are often associated with increased blood pressure (hypertension) and fast pulse (tachycardia). MIs involving the inferior (lower) wall of the left ventricle may result in abnormally slow pulse (bradycardia) and low blood pressure (hypotension). Abnormal cardiac rhythms manifested by irregular palpable pulses may be present and often indicate a highly critical situation. An S4 gallop sound may be heard when listening to the heart with the stethoscope (the S4 sound occurs just at the end of the relaxation or diastole phase of the ventricles, and is a sound caused by blood being squirted into an abnormal left ventricle by the left atrium). Signs of cardiac failure such as pulmonary (lung) congestion with the presence of audible rales (crepitations) at the lung bases, marked shortness of breath, and distended neck veins should be sought.
ELECTROCARDIOGRAM
An electrocardiogram (EKG) should be obtained in all cases of suspected MI. While an EKG is neither 100% sensitive nor 100% specific for MI, it is easily performed and without risk, and is very often of great value in determining the next step in treatment. and evaluation.
The earliest EKG finding during MI is elevation of the ST segments in the leads recording signals from the area of infarction (Fig 2) . ST segment elevation usually begins to occur within minutes of the onset of infarction, increases in degree over several hours, and may persist for up to 2 weeks after infarction. ST elevation may persist beyond 2 weeks in anterior wall MI, and may indicate a large area of abnormal ventricular wall motion (akinesis, dyskinesis) or ventricular aneurysm (a weakened area of the ventricular wall which tends to move paradoxically with contraction). The presence of ST segment depression in some leads with ST elevation in opposing leads (reciprocal changes) may indicate larger areas of infarction and are associated with a worse prognosis although such patients may benefit more greatly from interventions aimed at restoring coronary flow (recanalization).
As ST segment changes regress, T waves in leads with ST elevation become inverted and Q waves develop (Fig 3). These Q waves may become a permanent EKG feature in anterior wall infarctions, but may resolve in inferior wall infarctions.
Successful efforts at reestablishing coronary artery flow (recanalization) hasten the evolution of EKG changes, with ST changes resolving in minutes or hours instead of days or weeks.
ST segment elevation on EKG may occur in some non-MI disease states, particularly acute pericarditis. In such cases, it is critical to make the correct diagnosis, as fibrinolytic therapy (clot ‘busting’) is contraindicated in pericarditis, and may lead to severe complications.
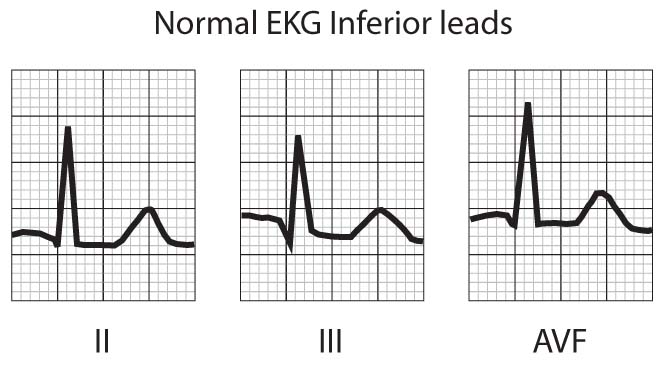
FIGURE 1 – NORMAL EKG PATTERN
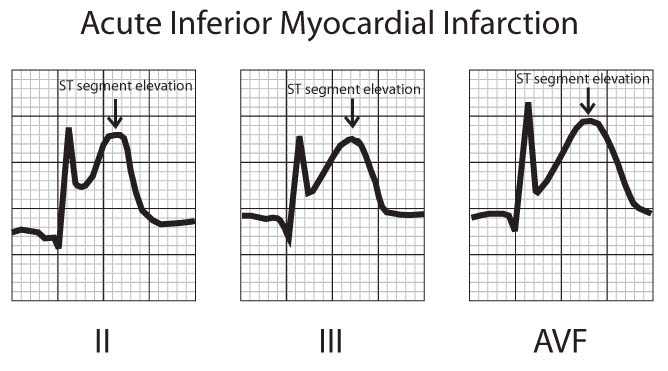
FIGURE 2 – ST SEGMENT ELEVATION DURING ACUTE MYOCARDIAL INFARCTION
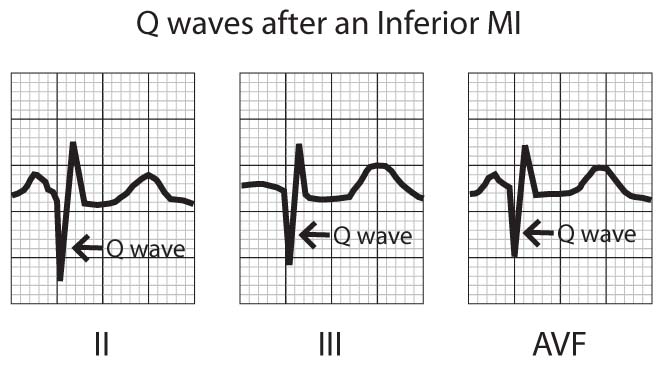
FIGURE 3 – Q WAVES ACUTE MYOCARDIAL INFARCTION

IMAGING STUDIES
While measurement of Troponin biomarkers are currently considered the most important finding in the diagnosis of acute myocardial infarction, imaging studies can provide very useful supplemental information.
The most common imaging study obtained in suspected acute MI is a plain film chest X-ray (CXR). There are no specific changes found on CXR with acute MI, however, alternative diagnoses for the patient’s symptoms may be suggested by CXR findings; for example, widening of the mediastinal structures as may occur with dissection of the aorta. In addition, findings of MI-related cardiac dysfunction may be present, such as fluid accumulation in the lung bases as a result of cardiac failure.
Coronary angiography is frequently used in the acute MI setting to assess coronary artery patency, and is still the gold standard for making the specific diagnosis of coronary artery disease as the basis for acute myocardial infarction. In addition, coronary angiography provides indispensable information in guiding the use of Percutaneous Coronary Intervention (PCI), or Coronary Artery Bypass Graft (CABG), to achieve reestablishment of coronary blood flow (reperfusion therapy). A catheter is placed in the root of the aorta at the origin of the coronary arteries, and radio-opaque dye is then flushed through the catheter. The resulting X-ray images (still and video) may be used to determine areas of occlusion within the arteries. Angioplasty or coronary artery bypass may then be performed to treat the occluded areas.
Echocardiography in the immediate post-MI period can provide very useful information regarding overall cardiac function, including left ventricular ejection capability, ventricular wall motion, and valve function, and may also reveal complications such as left ventricular aneurysm and ventricular thrombus. Echo may also be used to differentiate acute pericarditis from acute MI. Serial Echocardiographic exams may be utilized to assess cardiac response to recanalization and medical treatment in the weeks following acute MI.
MRI, CT and radionucleotide imaging studies are seldom used in the acute setting. More commonly, such studies may be utilized in the weeks or months following acute MI to aid in risk stratification. Thallium-201 and technetium-99m-sestamibi may be used alone or together to assess infarct size as well as myocardial perfusion and viability. CT scan and MRI imaging can be used to identify dissecting aortic aneurysm when this diagnosis is suspected. Contemporary multislice coronary computed tomography can be used to assess coronary artery disease in situations where a non-atherosclerotic cause for MI is being considered.
TREATMENT OF MYOCARDIAL INFARCTION
The primary goal of treatments for patients experiencing acute myocardial infarction is to reestablish blood flow through diseased coronary arteries (reperfusion therapy), either via finbrinolytic (clot buster) administration, the use of Percutaneous Coronary Intervention (PCI), and in some cases Coronary Artery Bypass Graft (CABG) surgery. Secondary goals include relief of ischemic pain, and assessment and correction of abnormalities in the hemodynamic state (overall cardiovascular stability).
More than 50% of MI related deaths occur in the first hour after onset of symptoms. These deaths are primarily caused by ventricular fibrillation (VF) related to myocardial ischemia. Up to 60% of patients in VF can be resuscitated by defibrillation if such treatment is administered by paramedics or bystanders on the scene (using automated external defibrillator – AED). Re-canalization therapy (removal of obstructing clot in the coronary artery by fibrinolytic therapy or angioplasty) has the best chance for success if administered within the first hour of symptom onset. Thus, prompt recognition of symptoms, rapid deployment of paramedics to the scene, and expeditious transport to hospital, are essential in increasing the chance for patient survival.
Once the Emergency Department has been reached, and the diagnosis of acute MI suspected by the history, physical exam and EKG, aspirin (to those without allergy or other contra-indications) in a dose of 162-325mg should be given orally to reduce the tendency for additional clot formation or extension within the coronary arteries. Heparin should be administered either intravenously or subcutaneously (depending on the form) for the same reason. Nitroglycerine increases coronary artery flow, and should be administered to patients with continuing chest pain. Chest pain non-responsive to nitroglycerine may be treated with small repetitive doses of intravenous morphine. Oxygen administered by mask or nasal cannula should be applied. Initiation of beta-blocker agents is usually indicated, particularly if hypertension is present, but must be used cautiously if there are signs of cardiac failure. Lines for intravenous fluid administration are typically placed, although rate of flow must be adjusted with consideration of hemodynamic state. Intravenous agents (such as lidocaine) may be required to correct rhythm disturbances.
SURGICAL TREATMENT
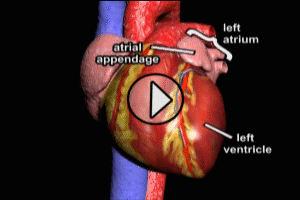
Coronary Artery Bypass Graft (CABG)
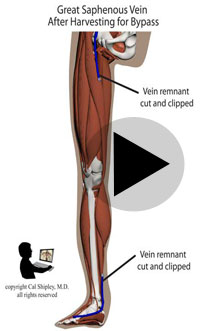
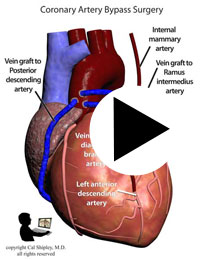
Starting in the early 1960s, surgeons have been performing CABG procedures to bypass the obstructed portions of coronary arteries in the immediate post-myocardial infarction period. The earliest such procedures involved attaching the patient’s internal mammary artery to the diseased coronary artery just beyond the point of arterial obstruction, thus reestablishing blood flow to the myocardium. As the technique evolved, veins harvested from the patient (usually from the lower limbs), were attached to from the aorta to the diseased artery. The biggest advantage of venous grafts was that multiple sections of harvested vein grafts could be used to bypass multiple diseased coronary arteries in patients with multi-vessel disease. Today, both vein grafts and internal mammary artery grafting may be used depending on the clinical situation of a given patient and surgeon preference.
After its introduction, Coronary Artery Bypass Grafting rapidly became the standard of care for individuals suffering myocardial infarction due to coronary artery disease. The development in the late 1970s of Percutaneous Coronary Intervention (PCI) techniques, and the advent of clot busting fibrinolytics in the 1980s, has reduced the use of coronary artery bypass grafting in the acute MI period, although it still has a role to play in certain patients. (Please see the section on “PCI versus CABG” below). The following series of illustrations depict coronary anatomy & disease, and bypass grafting:
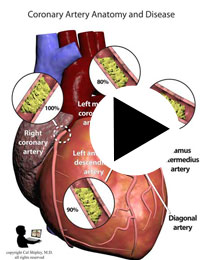
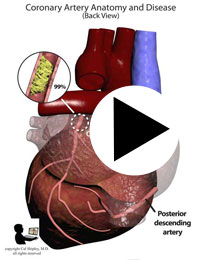
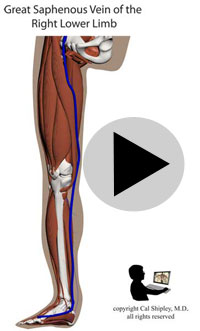
RECANALIZATION THERAPY
Early recanalization (removal of coronary artery clots) to permit re-perfusion of ischemic myocardium in ST segment elevation acute MI represents the most effective therapy for survival in these patients. The use of recanalization techniques has reduced overall mortality from acute MI by 10-25%. Recanalization may be accomplished either by administration of fibrinolytic (clot “busting”) therapy, or through Percutaneous Coronary Intervention (PCI) with angioplasty.
Fibrinolytic therapy
Several agents have been approved by the FDA for administration as fibrinolytic’s. Streptokinase (SK) and alteplase (t-PA) are shorter acting agents, and must be administered slowly over a period of 30-60 minutes and 90 minutes respectively. Two newer long-acting agents, reteplase (r-PA) and tenecteplase (TNK–t-PA), may be given in single or double bolus injections, respectively.
Coronary artery patency at 90 minutes after administration of these agents is better for t-PA, r-PA and TNK-t-PA (80%), than for SK (50-60%).
Time is of the essence with fibrinolytic therapy: 40 lives or more are saved per 1000 if the fibrinolytic is administered within the first hour, 20 to 30 lives saved per 1000 for hours 2 to 12, and a statistically nonsignificant 7 lives saved per 1000 for hours 13 to 24.
Younger patients tend to have better outcomes with fibrinolytic agents, as do those with an ischemic anterior wall compared to those with an ischemic inferior wall.
The most serious side effect of fibrinolytic agents is bleeding, and fatal cerebral hemorrhage in particular. Approximately 0.5-1.0 % of patients suffer this complication. The elderly, females, and those with hypertension (high blood pressure) are at higher risk for cerebral hemorrhage.
Primary Percutaneous Coronary Intervention
PCI is now the preferred recanalization therapy. PCI achieves re-opening of the occluded coronary artery by inflation of a balloon which is placed across the obstructed area by means of a catheter (angioplasty also known as Percutaneous transluminal coronary angioplasty or PTCA). This is often supplemented by placement of a mechanical stent to enlarge and maintain the coronary opening. Drug eluting stents (sirolimus, paclitaxel), are often used to reduce the rate of re-stenosis (re-occlusion), although such stents have been seen to slightly increase the risk of late thrombosis within the coronary artery.
PCI has been found to have statistically significant benefits over fibrinolytic agents, with lower mortality rates, and lower rates of nonfatal reinfarction and intracerebral hemorrhage. As with fibrinolytics, time is critical, and PCI performed within the first 1-2 hours of symptom onset yields the greatest benefit. However, even when administered between 12 and 48 hours of symptom onset, PCI can reduce infarct size, and possibly adverse events.
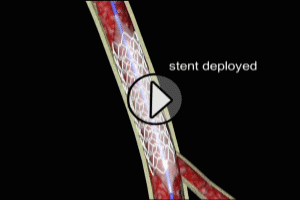
Choice of Recanalization Therapy
Provided there is adequate institutional and operator experience, PCI is the preferred recanalization therapy (with balloon angioplasty followed by stent placement). This particularly holds true when there are contraindications to the use of fibrinolytic agents, such as risk factors for, or history of, intracerebral hemorrhage, female gender, treatment for hypertension or age >70, and for patients presenting more than 3 hours after symptom onset.
However, in the absence of contraindications, and if the time from onset of symptoms is 3 hours or less, and the choice of PCI will delay therapy by one hour or more, then fibrinolytics are preferred.
PCI versus CABG
After several decades of experience using both CABG and PCI techniques to achieve reperfusion of coronary arteries in patients with acute myocardial infarction, conventional wisdom held that PCI was most useful in patients with “low complexity” coronary artery disease, defined as disease in 2 or less arteries, and no significant disease in the left main coronary artery.
In recent years, with the advent of improved stent technique and stent design, as well as drug-eluting (drug coated) stents, and the development of more effective anti-platelet (anti-coagulation) drugs, the landscape has begun to shift, with many studies indicating comparable results between CABG and PCI in higher complexity patients. In recent years, with the advent of improved stent technique and stent design, as well as drug-eluting (drug coated) stents, and the development of more effective anti-platelet (anti-coagulation) drugs, the landscape has begun to shift, with many studies indicating comparable results between CABG and PCI in higher complexity patients.
COMPLICATIONS OF MYOCARDIAL INFARCTION
HEART FAILURE
Cardiac failure (failure of the pumping action of the heart) is the leading cause of in hospital mortality from acute MI. Clinical manifestations of cardiac failure can include low blood pressure, weak pulse, cool extremities, a third heart sound, pulmonary congestion, oliguria (reduced urine output), and obtundation (impaired consciousness).
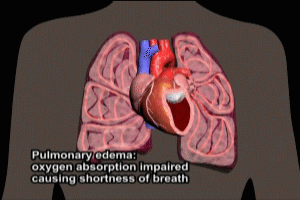
Left Ventricular Dysfunction and Cardiogenic shock
The degree of left ventricular dysfunction is directly related to infarct size. Signs of hemodynamic instability (shock) generally occur with involvement of 20-25% of left ventricular muscle mass, whereas involvement of 40% or more is associated with cardiogenic shock and high rates of mortality. The most common physical findings are S3 S4 gallops and pulmonary congestion. Recanalization therapy (PCI, finbrinolytics, or CABG – coronary artery bypass graft) is critical to prevention or reduction of cardiac failure in the early stages after MI, and should be initiated as soon as practicable. Medical therapy for cardiac failure consists of supplemental oxygen, use of diuretics, nitroglycerin to reduce cardiac preload, and ACE inhibitor therapy.
Cardiogenic shock is a severe form of left ventricular dysfunction characterized by severe hypotension (low blood pressure with systolic pressures <80mm Hg) and markedly reduced cardiac output (cardiac index <1.8L/min/meter squared). Infarct size typically involves 40% or more of the left ventricular muscle mass, and mortality rates are in the range of 70-80%. Early and urgent mechanical revascularization (PCI or CABG) is associated with the best chance for survival in these patients.
Placement of an Intra Aortic Balloon Pump (IABP) is recommended for treatment of cardiogenic shock. By means of a catheter, the deflated balloon is placed into the aorta just above the coronary artery openings. An electrocardiogram (EKG) triggers inflation of the balloon during early diastole (relaxation phase of the ventricles), and the balloon is then deflated during early systole (contraction phase of the ventricles). This effectively forces more blood flow through the coronary arteries, and reduces left ventricular afterload. Placement of an IABP can provide temporary stability for patients undergoing angiography and subsequent revascularization or other cardiac surgical procedures, as does not reduce mortality from cardiogenic shock when used alone. IABP is ineffective in the setting of severe aortic valve insufficiency or marked peripheral vascular disease.
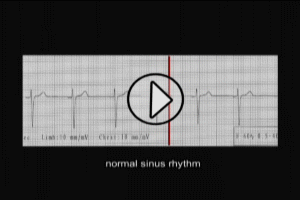
RHYTHM DISTURBANCES (ARRHYTHMIA)
Ventricular Arrhythmias
Ventricular fibrillation (VF) is the most serious arrhythmia associated with acute MI, and is a primary contributor to mortality within the first 24 hours. Within the first 4 hours of acute MI, the incidence of VF is 4-5%. The frequency of VF then rapidly declines over the first 24-48 hours. All patients should be continuously monitored for several days after diagnosis. Biochemical changes with acute MI, including myocardial ischemia, intracellular electrolyte disturbance, lipolysis (fat necrosis), increased output of adrenaline and, free fatty acid production all contribute to an environment which engenders arrhythmias such as VF. In addition, production of oxygen free radicals with recanalization therapy may also trigger VF.
So called “tacharrhythmias”, such as ventricular tachycardia (VT), are also commonly seen in the immediate post-MI period, and may cause serious hemodynamic instability (shock)..
Late VF, defined as VF occurring >48 hours after onset of acute MI, often indicates larger areas of infarct, or cardiac failure, and is a worrisome prognostic sign. Aggressive measures, such as placement of an implantable cardioverter-defibrillator (ICD), are justified.
Electrical cardioversion (defibrillation) is the treatment of choice for VF or ventricular tachycardia (VT) which is causing hemodynamic compromise (shock). Slower VT, in patients who are hemodynamically stable, may be treated chemically with agents such as intravenous amiodarone or intravenous lidocaine.
Patients with late or recurrent VF or VT may be stabilized long term with placement of an implantable cardioverter-defibrillator (ICD). ICD appears to give greater benefit than anti-arrhythmic medications in such patients.
Atrial Fibrillation
Atrial fibrillation occurs in 10-15% of patients within 24 hours of acute myocardial infarction. The likelihood of Atrial fibrillation is increased by larger infarct area, heart failure, pericarditis, atrial infarction, hypoxia (reduced serum oxygen levels), pre-existing lung disease, hypokalemia, hypomagnesemia, hyperadrenergic states, and age. AF with rapid ventricular response rates may cause hemodynamic compromise, and systemic embolism (ejection of clot into the systemic circulation) is also a risk, so prompt anticoagulation with heparin is recommended.
Treatment for AF includes electrical cardioversion for patients with hemodynamic compromise, intravenous digoxin for rate control in patients with rapid ventricular response and ventricular dysfunction; an intravenous beta blocker to control ventricular rate when ventricular dysfunction is absent, or intravenous verapamil or diltiazem for those who cannot tolerate beta-blockers. Initial anticoagulation with heparin is recommended for all patients with AF.
Bradycardias and Heart Block
Bradycardia (abnormally slow heart rate) and dysfunction of the Sinus and AV (atrio-ventricular) node (areas which initiate cardiac chamber contraction) are common in acute myocardial infarction. Bradycardia is often seen in inferior wall acute MI (30-40% of patients), and within the first hour of acute MI. The Bezold-Jarisch reflex consists of bradycardia occurring with recanalization of the right coronary artery. Treatment of bradycardia with anticholinergic agents, such as atropine, are indicated in symptomatic patients only (typically heart rate <50).
New onset sinus or AV node blocks, Bundle Branch Blocks, and intra-ventricular conduction delays are associated with a poor prognosis. Mortality in these situations is related more to the extent of myocardial damage than the effects of the blocks on cardiac pacing. AV blocks are categorized according to their severity as first, second, or third degree, while bundle branch blocks are named according to which bundle is affected (ie – LBBB- left bundle brach block, RBBB – right bundle branch block, or bifasicular block) and whether the block is partial or complete.
For patients in the immediate post-MI period in whom heart blocks are symptomatic, temporary pacing solutions such as patch electrodes or transcutaneous pacemakers can be utilized. Permanent pacemakers are recommended for any second- or third-degree AV block in association with BBB and symptomatic AV block at any level.
RIGHT VENTRICULAR INFARCTION
While the majority of acute MIs involve the left ventricle, occlusions occurring in the proximal right coronary artery (before the branches to the right ventricle), can result in right ventricle (RV) infarction. 10-15% of inferior wall MIs display features of RV infarct. Hypotension (low blood pressure) with clear lung fields and elevated jugular venous pressure in patients with inferior or inferio-posterior MIs should raise the suspicion of RV infarct. Distention of the jugular vein on inspiration (Kussmaul’s sign) is often seen, as is ST segment elevation in the right-sided EKG leads. Echocardiography is useful in making the diagnosis, showing RV dilation and dysfunction. Swan-Ganz catheter measurements typically show a right atrial pressure (RAP) of 10mm Hg or > and the RAP is 80% or more of the capillary wedge pressure.
Treatment for RV infarct consists of early recanalization, IV fluid therapy to maintain RV preload pressures, reduction of LV afterload (consider IABP), and avoidance of diuretics, and venodilators (such as nitroglycerine). If fluid loading fails to improve cardiac output, ionotropic agents such as Dobutamine may be administered. High grade AV blocks are common, and may be treated with temporary pacing. AF should be promptly cardioverted, as it may cause severe hemodynamic instability.
MECHANICAL COMPLICATIONS
Mechanical complications, such as acute mitral valve regurgitation, ventricular septal defect, free wall rupture, and LV aneurysm, usually occur within the first weeks after acute MI, and account for approximately 15% of MI-related deaths. These complications are generally heralded by the appearance of a new murmur, or sudden hemodynamic deterioration. Echocardiography (transthoracic or transesophageal) are useful diagnostic tools, as is a Swan-Ganz catheter (balloon flotation catheter).
Most mechanical complications require urgent surgical repair.
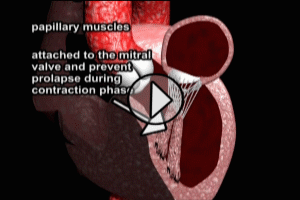
THROMBUS OF THE LEFT VENTRICLE
Formation of mural thrombus (blood clot) in the Left Ventricle is more common in large ST segment elevation anterior wall infarcts and heart failure. When the thrombus can be seen on echocardiogram, the risk of embolism (travel of clot into the systemic circulation) is particularly high. Therefore, all patients with an ST segment elevated anterior infarct should have echocardiography performed.
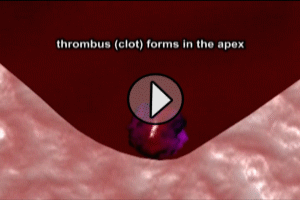
Previously, thromboembolism was thought to be responsible for up to 25% of deaths post-MI, but this percentage has dropped with greater use of recanalization therapy and anticoagulants. Mural thrombi may embolize to virtually any portion of the systemic arterial circulation, often with devastating effects, especially if the cerebro-vascular circulation is affected.
Cal Shipley, M.D. copyright 2020